Hanmi Brings New Hope to Patients with Congenital Hyperinsulinism
Hanmi Brings New Hope to Patients with Congenital Hyperinsulinism
Hanmi Presents Findings at ESPE & ESE 2025
Interim Phase 2 Results for Efpegerglucagon Show Favorable Safety and Strong Efficacy
Efpegerglucagon: World’s First Once-Weekly Therapy in Development for Congenital Hyperinsulinism (CHI)
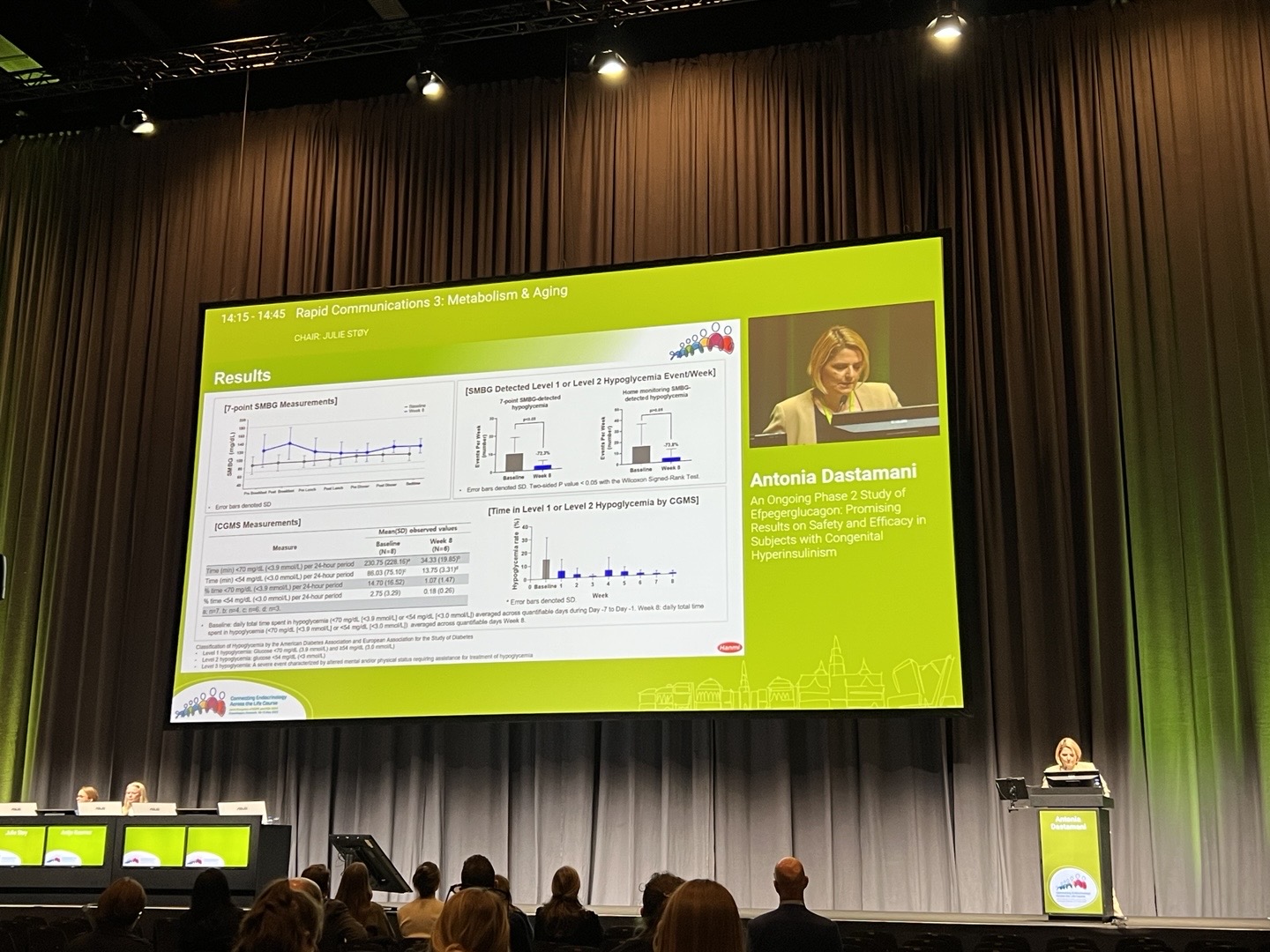
Dr. Antonia Dastamani, a pediatric endocrinologist, presented interim Phase 2 clinical trial results of efpegerglucagon at the Joint Congress of the European Society for Paediatric Endocrinology (ESPE) and the European Society of Endocrinology (ESE) on May 11 in Copenhagen, Denmark.
(May 26, 2025) Hanmi Pharmaceutical is making steady progress in the development of efpegerglucagon (HM15136), a novel treatment for congenital hyperinsulinism (CHI).
Hanmi Pharmaceutical announced on May 26 that it had presented interim analysis results from the Phase 2 clinical trial of efpegerglucagon at the Joint Congress of the European Society for Paediatric Endocrinology (ESPE) and the European Society of Endocrinology (ESE), held in Copenhagen from May 10 to 13. The data were shared through both oral and poster sessions.
Congenital hyperinsulinism is a rare disease characterized by excessive insulin secretion, leading to recurrent severe hypoglycemia. It occurs in approximately 1 in every 25,000 to 50,000 births, with about 300 new diagnoses annually in the United States and Europe. Although one treatment has been approved, its efficacy is limited to specific genotypes and is frequently associated with significant side effects, including hypertrichosis, fluid retention, and heart failure. Consequently, many patients turn to off-label therapies or resort to pancreatectomy.
Hanmi is developing efpegerglucagon as a potential breakthrough therapy to address critical unmet needs in CHI treatment and to overcome the limitations of existing options. A global Phase 2 clinical trial is currently underway across five countries. At the conference, Hanmi presented interim results from Cohort 1 of the ongoing Phase 2 clinical trial, involving eight patients who received efpegerglucagon over an eight-week treatment period. The data showed that efpegerglucagon was well-tolerated, with no significant safety issues identified in vital signs, physical examinations, laboratory tests, or electrocardiograms. No adverse events requiring treatment discontinuation or raising particular safety concerns were reported.
Over the course of the treatment period, both the frequency and duration of hypoglycemia (blood glucose <70 mg/dL) and severe hypoglycemia (blood glucose <54 mg/dL) decreased significantly. Notably, by week 8, episodes of hypoglycemia below 70mg/dL had decreased by 72.3% and episodes of severe hypoglycemia below 54mg/dL had decreased by 87.5%.
The mean half-life of the drug at week 8 was found to be approximately 89 hours, supporting the once-weekly dosing regimen. These results suggest that efpegerglucagon has the potential to significantly reduce disease burden by providing sustained efficacy, favorable safety, and enhanced dosing convenience for patients.
Dr. Antonia Dastamani, a pediatric endocrinologist from the UK’s Great Ormond Street Hospital, stated, “efpegerglucagon has shown encouraging potential to reduce the risk of hypoglycemia with just once-weekly dosing. We hope this therapy will be developed swiftly to offer a new solution for patients with this rare disease.”

Pediatric patients and their caregivers posed for a commemorative photo at the conference hosted by Congenital Hyperinsulinism International (CHI) on May 9 in Copenhagen, Denmark.
Meanwhile, Hanmi participated in the Congenital Hyperinsulinism International (CHI) Family Conference, held from May 8 to 10 in Copenhagen. During the event, the company directly engaged with young CHI patients and their families, demonstrating support for their challenges and reaffirming its commitment to developing innovative treatment.
Established in 2005, CHI is a nonprofit organization that hosts regular family gatherings, bringing together healthcare professionals, researchers, and industry leaders to exchange the latest advances and expand global collaboration in the field.
CHI primarily affects newborns due to genetic abnormalities and can lead to life-threatening, persistent hypoglycemia. Without proper treatment, the condition may cause irreversible brain damage, placing tremendous emotional and physical strain on patients and their families.
Hanmi Pharmaceutical has been a supporter of CHI since 2020, helping pediatric patients and their families find comfort, courage, and hope for a cure.
Dr. Moon Hee Lee, Head of the clinical team at Hanmi Pharmaceutical, highlighted the significance of the interim results, stating, “We are encouraged by the positive clinical data of efpegerglucagon, which is showing strong efficacy and favorable safety in CHI patients.” She added, “We will do our best in clinical development to ensure that efpegerglucagon can be a new treatment that significantly improves the quality of life for patients suffering from this rare disease.”
<The End>