Hanmi to Advance Next-Generation IL-2 Combination Trial with KEYTRUDA® (pembrolizumab) in Collaboration with MSD
Hanmi to Advance Next-Generation IL-2 Combination Trial with KEYTRUDA® (pembrolizumab) in Collaboration with MSD
Hanmi announces collaboration with MSD to Evaluate HM16390 in Combination with KEYTRUDA
Initiates Phase 1 Clinical Trial for Patients with Advanced or Metastatic Solid Tumors
Optimized IL-2 Receptor Binding Maximizes Efficacy While Reducing Adverse Effects
Hanmi Pharmaceutical has entered into a clinical trial collaboration and supply agreement with MSD (Merck & Co., Inc., Rahway, NJ, USA) to evaluate the safety and efficacy of Hanmi’s LAPS IL-2 analog, HM16390 in combination with MSD’s anti-PD-1 therapy, KEYTRUDA® (pembrolizumab) in a Phase 1 clinical trial. Hanmi will sponsor the clinical trial, and MSD will supply KEYTRUDA.
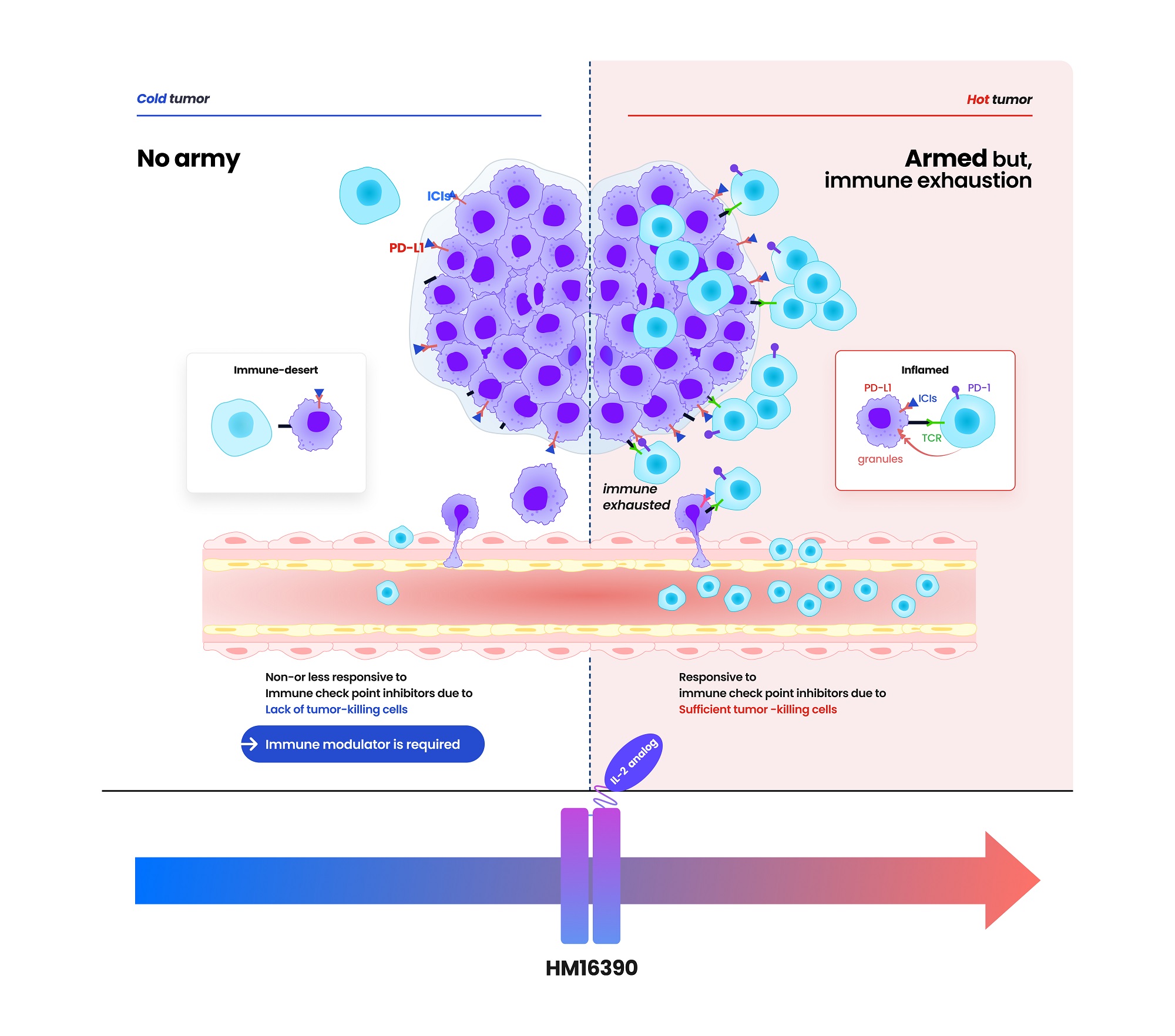
HM16390 is a next-generation IL-2 variant designed with a differentiated strategy to regulate the differentiation and proliferation of immune cells. By inducing T-cell proliferation and activation, HM16390 increases the number of tumor-infiltrating lymphocytes (TILs) within the tumor microenvironment (TME), enhancing the response to immune checkpoint inhibitors. This mechanism helps convert immunologically “cold tumors” into “hot tumors,” thereby maximizing antitumor efficacy.
Currently, the approved recombinant IL-2 therapy, PROLEUKIN® (aldesleukin) is recommended only for limited clinical use due to safety concerns. While most IL-2 analogs in development primarily focus on modulating IL-2 beta receptor binding affinity, this approach has presented limitations in terms of safety.
Reducing IL-2 beta receptor binding affinity lowers the risk of vascular leak syndrome (VLS) but also diminishes antitumor efficacy. In contrast, increasing IL-2 beta receptor binding affinity while eliminating IL-2 alpha receptor binding enhances antitumor effects but significantly raises the risk of cytokine release syndrome (CRS) and other severe adverse effects.
To overcome these limitations, HM16390 has adopted a differentiated development strategy. Unlike existing IL-2 candidates, HM16390 precisely modulates IL-2 alpha receptor binding to enhance safety while maximizing efficacy. The potential of the approach is to maintain antitumor effects while minimizing severe adverse effects.
At the 2024 Society for Immunotherapy of Cancer (SITC) Annual Meeting, Hanmi presented the differentiated development strategy of HM16390, along with preclinical data showing complete tumor remission in animal studies. As a result, there is growing interest in its future research and clinical development.
HM16390 is an immuno-oncology therapy developed using Hanmi’s LAPSCOVERY platform, which enhances efficacy, safety, and durability. Designed as a long-acting treatment, HM16390 allows for once-per-treatment cycle subcutaneous (SC) administration in cancer therapy. Hanmi is developing HM16390 not only as a monotherapy but also in combination with immune checkpoint inhibitors for the treatment of various solid tumors and is currently conducting a global Phase 1 clinical trial.
Dr. Jongchul Park, professor at the Head and Neck Cancer Center at Harvard Medical School, Massachusetts General Hospital (MGH), and principal investigator for the phase 1 clinical trial in the United States and Korea, stated, “Through this collaboration with MSD, we anticipate that the combination of HM16390 and KEYTRUDAwill improve treatment outcomes for patients with advanced or metastatic solid tumors and lead to meaningful clinical advancements.”
This agreement marks Hanmi’s third collaborative agreement with MSD, following previous agreements for the PD-L1/4-1BB bispecific antibody, BH3120, and the small molecule CCR4 antagonist, tivumecirnon. Hanmi, already recognized as the leader in obesity drug development, is now demonstrating its strong R&D capabilities and competitive oncology pipeline.
Young Su Noh, Director/Head of ONCO Clinical Research and Development at Hanmi Pharm. Co., Ltd., stated, “Hanmi possesses a differentiated pipeline in oncology, particularly in immunotherapy. Throughout the year, we will continue to present our research achievements at various scientific conferences.”
KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
■ Contact info:
Official Websites: www.hanmipharm.com
innovation@hanmi.co.kr, +08-2-410-0467